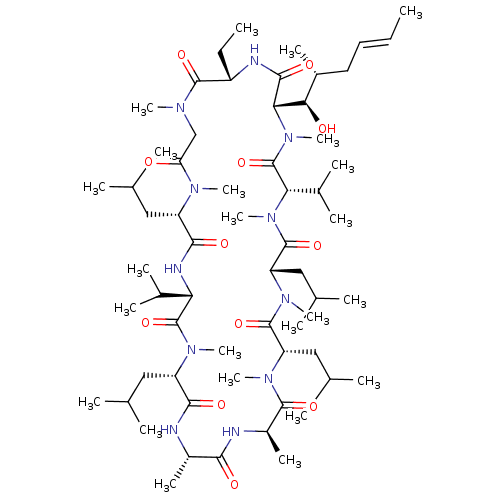
BDBM50022815 (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(1R,2R,4E)-1-hydroxy-2-methylhex-4-en-1-yl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-bis(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecone::30-Ethyl-33-((E)-1-hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,28-octamethyl-1,4,7,10,13,16,19,22,25,28,31undecaaza-cyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone::30-Ethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31undecaaza-cyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone::CYCLOSPORINE::Cyclosporin A::Cyclosporine A::Cyclosproine A::US10077289, Compound Cyclosporin A::US9138393, Cyclosporin A
SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C
InChI Key InChIKey=PMATZTZNYRCHOR-CGLBZJNRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50022815
Found 9 hits for monomerid = 50022815